Answer: Option (d) is the correct answer.
Explanation:
When a base completely dissociate into ions when dissolved in a solution then it is known as a strong electrolyte.
For example,
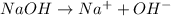
Whereas, a solution that contains large number of ions is known as a strong electrolyte.
Thus, we can conclude that for a strong base it is true that it is a strong electrolyte.