Answer : A thermochemical equation gives a balanced stoichiometric chemical equation which mainly includes the enthalpy change during the reaction process, ΔH.
Combustion reactions are those reactions which occurs when a hydrocarbon molecule reacts with oxygen to give products as carbon dioxide and water. In other words, combustion reaction involves a reaction between any combustible material and an oxidizer to form an oxidized product.
For combustion of methane, methane reacts with the oxygen and forms carbon dioxide and water as the products.
The thermochemical equation will be as
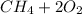 →
→
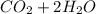 . and ΔH = -891 KJ/mol
. and ΔH = -891 KJ/mol