Answer:
B.
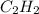
Step-by-step explanation:
The
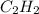 is the only molecule that has covalent bonds (bond that occurs between two nonmetals), this means that the difference in electronegativity, of the molecule, is small, therefore it will not form a dipole.
is the only molecule that has covalent bonds (bond that occurs between two nonmetals), this means that the difference in electronegativity, of the molecule, is small, therefore it will not form a dipole.
Without dipole, the interaction is weaker, so less energy (heat) is required to break the covalent bonds than is required to break an ionic bond.
The other molecules have ionic bonds.