Answer: The blanks are completed below.
Step-by-step explanation:
Enthalpy change of the reaction is defined as the difference in the potential energy of the products and the potential energy of the reactants. It is represented as

Mathematically,
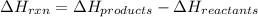
When
 of the reaction is negative, then potential energy of the products is less than the potential energy of the reactants. So, energy is being released.
of the reaction is negative, then potential energy of the products is less than the potential energy of the reactants. So, energy is being released.
Thus, a +ve
 means that energy is released from a reaction.
means that energy is released from a reaction.
When
 of the reaction is positive, then potential energy of the products is more than the potential energy of the reactants. So, energy is being absorbed.
of the reaction is positive, then potential energy of the products is more than the potential energy of the reactants. So, energy is being absorbed.
Thus, a -ve
 means that energy is absorbed from a reaction.
means that energy is absorbed from a reaction.