Step-by-step explanation:
Number of moles is defined as mass divided by molar mass.
Mathematically, No. of moles =
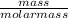
It is given that there are 6.80 moles and it is known that molar mass of water is 18 g/mol.
Therefore, calculate mass as follows.
No. of moles =
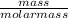
6.80 mol =
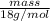
mass = 122.4 g
Thus, we can conclude that mass of 6.80 moles
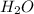 is 122.4 g.
is 122.4 g.