Answer:
 is a acid.
is a acid.
Explanation: Acid is anything which donates
 ions and in this reaction
ions and in this reaction
 is donating
is donating
 ions, therefore it is considered as an Acid.
ions, therefore it is considered as an Acid.
This reaction is a type of reaction an acid undergoes that is when an acid reacts with metal it produces metal salt and hydrogen gas
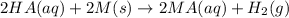
and the given reaction is
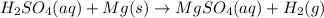
where,
 = Acid
= Acid
Mg = metal
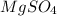 = Metal Salt
= Metal Salt
 = Hydrogen gas
= Hydrogen gas