Answer: Option (b) is the correct answer.
Step-by-step explanation:
Movement of molecules of a substance is dependent on temperature also. This is because energy obtained by an object due to its motion is known as kinetic energy.
Also,
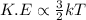
Hence, kinetic energy is directly proportional to temperature which means that when there is increase in temperature then there will occur an increase in molecular movement of the substance.
Similarly, a decrease in temperature will lead to a decrease in molecular movement of the substance.
Thus, we can conclude that temperature is a measure of molecular movement .