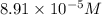Answer: The concentration of sulfate ions in the solution is
Step-by-step explanation:
To calculate the number of moles for given molarity of solution, we use the equation:
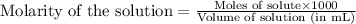 .......(1)
.......(1)
For barium ions:
Molarity of solution = 0.00100 M
Volume of solution = 5.00 mL
Putting values in equation 1, we get:
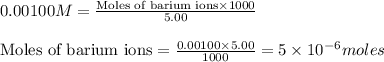
The chemical equation for the ionization of barium sulfate follows:
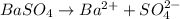
By Stoichiometry of the reaction:
1 mole of barium ions precipitate 1 mole of sulfate ions
So,
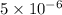 moles of barium ions will precipitate
moles of barium ions will precipitate
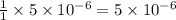 moles of sulfate ions
moles of sulfate ions
Now, calculating the molarity of sulfate ions by using equation 1:
Moles of sulfate ions =
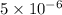 moles
moles
Volume of solution = 56.1 mL
Putting values in equation 1, we get:
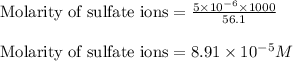
Hence, the concentration of sulfate ions in the solution is