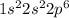Answer : The ground-state electronic configuration of oxide ion is,
Explanation :
Electronic configuration : It is defined as the distribution of electrons of atom in an atomic orbital.
Oxygen
The total number electrons in oxygen = 6
The electronic configuration of oxygen is,
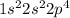
Oxide ion
The total number electrons in oxide ion,
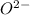 = 6 + 2 = 8
= 6 + 2 = 8
The charge on O is (-2). So, we are adding 2 electrons.
The electronic configuration of oxide ion is,