Answer:
The root mean square velocity of ozone molecule at temperature 1091 k is 752.9 m/s
Step-by-step explanation:
Atmospheric temperature high above the stratosphere is 1091 k. We have to find the root mean square velocity of the ozone molecule at that temperature.
Mathematically, the rms velocity is given by :
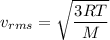
where
R is the gas constant
T is the temperature
M is the molar mass, for ozone M = 0.048 kg/mole
So,
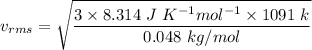
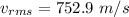
Hence, this is the required solution.