Answer: The shape of boron in borohydride ion
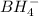 is tetrahedral and in boron trifluoride
is tetrahedral and in boron trifluoride
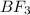 is trigonal planar.
is trigonal planar.
Explanation: Formula used
![:{\text{Number of electron pairs}} =(1)/(2)[V+N-C+A]](https://img.qammunity.org/2019/formulas/chemistry/high-school/7st3gx54vzpf9dydg9dxb1jwp6u02x4vxv.png)
where, V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
1.
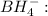
![{\text{Number of electrons}} =(1)/(2)[3+4-0+1]=4](https://img.qammunity.org/2019/formulas/chemistry/high-school/a934uq0cv06gsumteizsxewmdsv48f33jn.png)
The number of electron pairs is 4 that means the hybridization will be
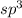 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral.
2.
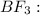
![{\text{Number of electron pairs}} =(1)/(2)[3+3-0+0]=3](https://img.qammunity.org/2019/formulas/chemistry/high-school/d4ekuinwb5cumiggcz3b45j79y5n7klicv.png)
The number of electron pairs is 3 that means the hybridization will be
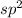 and the electronic geometry of the molecule will be trigonal planar.
and the electronic geometry of the molecule will be trigonal planar.