Hello!
The reaction that took place was
B) exothermic. The equation for the ΔH is
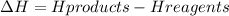
To know if this reaction is
exothermic or
endothermic we will need to look at the
sign of the
ΔH. If it is
positive, it means that enthalpy of the products is
higher than the enthalpy of the reagents and the reaction is
endothermic.
If the sign is negative, it means that the enthalpy of the products is
lower than the enthalpy of the reagents and the reaction is
exothermic. In this case, the sign is negative, so the reaction is exothermic.
Have a nice day!