Hello!
The reaction that the graph represents is
A. Exothermic because Hrxn=-167 kJTo calculate Hrxn we apply the following equation:
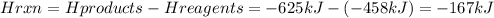
Looking at the graph, and at the result of the calculations, we can see that the enthalpy of the products is
lower than the enthalpy of the reagents, because the sign is negative. That means that the reaction
releases energy in the form of heat and that the reaction is
exothermic.
Have a nice day!