Answer:
7.56 × 10⁻⁵
Step-by-step explanation:
Let's consider the solution of the poorly soluble salt AB₃.
AB₃(s) ⇄ A³⁺(aq) + 3 B⁻(aq)
The molar solubility of the salt (S) is:
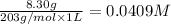
In order to relate S to the solubility product (Ksp), we will use an ICE Chart.
AB₃(s) ⇄ A³⁺(aq) + 3 B⁻(aq)
I 0 0
C +S +3S
E S 3S
The solubility product is:
Ksp = [A³⁺].[B⁻]³ = S . (3S)³ = 27 S⁴ = 27 (0.0409)⁴ = 7.56 × 10⁻⁵