Answer : The acid-base equilibrium reaction between
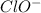 and
and
 will be,
will be,
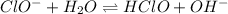
Explanation :
Acid-base equilibrium reaction : It is defined as a state of chemical equilibrium that exists when the both species of conjugate acid-base pair are present in the solution.
In the reaction, an acid donates a proton to a base and to produce a conjugate acid and a conjugate base in the solution.
The acid-base equilibrium reaction between
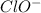 and
and
 will be,
will be,
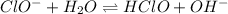
In this reaction,
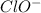 is a base and
is a base and
 is an acid that donates a proton to a base to produce
is an acid that donates a proton to a base to produce
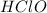 as a conjugate acid and
as a conjugate acid and
 as a conjugate base.
as a conjugate base.