Explanation :
Wave-particle duality tells us that wave and particle models apply to all objects.
We know that the De Broglie wavelength is given by :
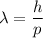
Where,
h is the Planck's constant
and p is the momentum.
For macroscopic objects, the mass of the object is more as compared to microscopic objects.
There is an inverse relationship between the momentum and the wavelength. So, the wavelength of macroscopic objects is short.
So, we do not observe wave properties in macroscopic objects because their wavelength is extremely short (undetectable).