Answer:
K2HPO4 = 447.0 mL
KH2PO4 = 353.0 mL
Step-by-step explanation:
A buffer is a solution when an acid, or a base, is in equilibrium with its conjugate base, or acid, and, because of that, when other acid or base is added to the solution, the pH remains almost unaltered.
The pH of a buffer can be calculated by the Handerson-Halsebach equation:
pH = pKa + log[A-]/[HA]
Where pKa = -logKa, Ka is the equilibrium constant of the acid, A- is the conjugate base and [HA] is the acid.
In this case, there are two salts presented K2HPO4 and KH2PO4, which will be dissociated and form the ions HPO₄²⁻ and H₂PO₄⁻, which come from the acid H₃PO₄ a polyprotic acid. This acid has 3 values of pKa:
H₃PO₄ ⇄ H⁺ + H₂PO₄⁻ (pKa1 = 2.15)
H₂PO₄⁻ ⇄ H⁺ + HPO₄²⁻ (pKa2 = 7.10)
HPO₄²⁻ ⇄ H⁺ + HPO₄³⁻ (pKa3 = 12.40)
In this case, the equilibrium presented is the second, H₂PO₄⁻ is the acid and HPO₄²⁻ is the conjugate base. Thus,
7.00 = 7.10 + log[HPO₄²⁻]/[H₂PO₄⁻]
log[HPO₄²⁻]/[H₂PO₄⁻] = -0.10
[HPO₄²⁻]/[H₂PO₄⁻] =
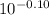
[HPO₄²⁻]/[H₂PO₄⁻] = 0.79
The concentration of the ions is the same as the salts. If they are combined in an equimolar solution, it means that the number of moles of the is the same. The concentration is the number of moles divided by the volume. Thus, calling K2HPO4 as 1 and KH2PO4 as 2:
(n1/V1)/(n2/V2) = 0.79
(n1/V1)*(V2/n2) = 0.79
Because n1 = n2,
V2/V1 = 0.79
V2 = 0.79V1
The total volume must be 800.0 mL, so
V1 + V2 = 800.0
V1 + 0.79V1 = 800.0
1.79V1 = 800.0
V1 = 447.0 mL
V2 = 353.0 mL