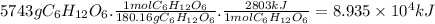Answer:
8.935 × 10⁴ kJ
Step-by-step explanation:
Let's consider the thermochemical equation for photosynthesis.
6 H₂O(l) + 6 CO₂(g) → C₆H₁₂O₆(s) + 6 O₂(g) ΔH = +2803 kJ/mol
2803 kJ of energy are absorbed per 1 mole of C₆H₁₂O₆. Considering the molar mass of C₆H₁₂O₆ is 180.16 g/mol, the energy required to produce 5743 g of C₆H₁₂O₆ is: