Answer:
Ion-dipole attraction force pulls
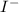 into solution
into solution
Step-by-step explanation:
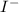 is an anion and water molecule is dipolar due to polarization of bonded electron towards more electronegative oxygen atom.
is an anion and water molecule is dipolar due to polarization of bonded electron towards more electronegative oxygen atom.
Hence, when KI is added in water, water molecule attracts
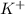 ions through its negatively charged polarized end and
ions through its negatively charged polarized end and
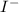 through its positively charged polarized end.
through its positively charged polarized end.
At equilibrium, this ion-dipole attraction force overcome the ionic interaction present between
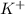 and
and
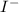 .
.
Hence KI become soluble into water.