- Pure substances that cannot be divided into simpler substances either by chemical or physical means are known as elements. They are made up of entirely one type of atom.
Thus, hydrogen and oxygen that is
 and
and
 are elements as they are made up of entirely one type of atom.
are elements as they are made up of entirely one type of atom.
Pure substance which is made up of two or more different elements combine in a fixed ratio are known as compounds. They can be separated into smaller substances.
Thus, water that is
 is a compound as it is made up of two or more different elements.
is a compound as it is made up of two or more different elements.
Hence, option B is correct that is:
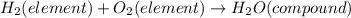
- The molarity of the solution is given as:
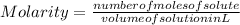 -(1)
-(1)
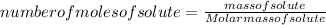
Molar mass of
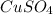 =
=
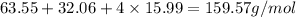
Molarity =
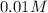 (given)
(given)
Volume of solution =
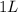 (given)
(given)
Substituting the formula of number of moles of solute in molarity formula:
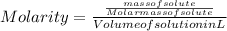
Substituting the values:
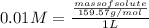
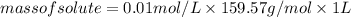
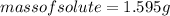
Hence, option B is correct.