Equivalence point in an acid base titration is the point when there are equal moles of acid and base in the solution.
The pH at the equivalence point of a strong acid and a strong base will be 7 as the solution will be neutral. The conjugate acid and the conjugate base of a strong base and strong acid will be neutral.

The pH at the equivalence point of a titration of weak acid and a strong base will be slightly basic as the conjugate base of the weak acid formed in the titration will be basic.

The pH at the equivalence point of a titration of strong acid and a weak base will be slightly acidic because the conjugate acid of the weak base is slightly acidic.

The pH at the equivalence point of the titration of a weak acid and a weak base is 7. But the pH changes are not sharp as both the acid and base are weak.
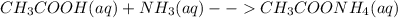
Therefore, the correct answer is B: Strong acid versus weak base