Answer:
V=44.8L
Step-by-step explanation:
Hello,
This problem can be solved by using the ideal gas equation:
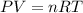
Nonetheless, we've got two different gases, nitrogen and neon, whose total moles turn out into 2 mol and the STP conditions which are 1 atm and 0 ºC, thus, the volume that accounts for the container's size is computed via:
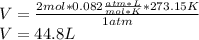
Best regards.