The combined gas law equation is as follows;
PV = nRT
where P - pressure
V - volume, n - number of moles, R - universal gas constant, T - temperature
number of moles are 0.654 mol, Volume - 12.30 L, Pressure is 1.95 atm
R - 8.314 Jmol⁻¹K⁻¹
Pressure is given in atm, we have to convert it to SI units - Nm⁻²
1 atm - 101 325 Nm⁻²
Therefore 1.95 atm = 101 325 x 1.95 = 197 584 Nm⁻²
Substituting these values into the equation,
T =
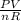
T =
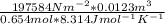
T = 2430.28/5.44
= 447 K