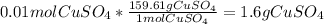Correct answer: C) 1.6 g
Molarity is the moles of solute present per liter solution.
The given molarity of the solution is 0.01 M.
Volume of the solution = 1 L
Calculating the moles from molarity and volume:
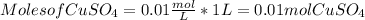
Converting moles to mass of copper sulfate: