Hello!
You need to calculate the volume of the container. To calculate the volume of this amount of N₂ gas we need to make the assumption that N₂ behaves like an
ideal gas.
1 mole of an ideal gas under Standard Temperature and Pressure occupies
22,4 L so the calculations are as follows:
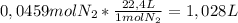
So, the volume of the container should be
1,028 L or more.
Have a nice day!