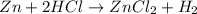Answer: C) neutralization
Step-by-step explanation:
A. Reduction is a type of chemical reaction in which substance is made to gain electrons or gain hydrogen.
Example:
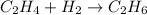
B. Combustion is a type of chemical reaction in which fuel is reacted with oxygen to form carbon dioxide and water.
Example:
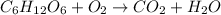
C. Neutralization reaction is defined as the chemical reaction in which an acid reacts with a base to produce a salt and water molecule.
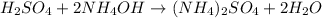
D. Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
Example: