Answer:
C) Oxygen-18 contains 10 neutrons while oxygen-16 only contains 8 neutrons.
Step-by-step explanation:
As we know that isotopes are two different types of same atom
In isotopes the number of neutrons must be different while atomic number or the number of protons may be different
So we know that
in oxygen-16 atom we have
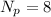
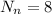
while in oxygen-18 atom we have
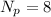
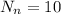
So correct answer will be
C) Oxygen-18 contains 10 neutrons while oxygen-16 only contains 8 neutrons.