Answer: 2 grams will be remaining.
Explanation: Half life is the time in which the amount of radioactive substance remains half.
For example if half life of a radioactive substance is 3 hours and we have 16 grams of it then in two hours, 8 grams will be remaining. In next two hours, 4 grams will be remaining. Similarly, in next two hours, 2 grams and further in next two hours 1 gram of the substance will be remaining.
For the given problem, the half life of the radioactive substance is 4 hours. It means the initial amount of this substance will remain half in 4 hours.
Originally we have 8 grams of the radioactive substance. In first 4 hours, 4 grams of the substance will be remaining. Now, in next 4 hours that is in total 8 hours, 2 grams of the substance will be remaining.
These problems could also be solved mathematically using the formula:
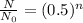
where,
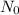 is the initial amount of the substance, N is remaining amount and n is the number of half lives.
is the initial amount of the substance, N is remaining amount and n is the number of half lives.
number of half lives(n) =
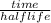
From given information, time is 8 hours and half life is 4 hours.
So, n =
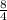 = 2
= 2
Initial amount is given as 8 grams and it asks to calculate the remaining amount. Let's plug in the values in the equation:
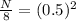
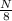 = 0.25
= 0.25
N = 8(0.25)
N = 2
So, from both ways 2 grams of the radioactive substance will be remaining after 8 hours.