Answer:
The proper equilibrium-constant expression for the equation is:
![K_(eq)=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2019/formulas/chemistry/college/ipsl1v22rc3t975zd5uni7ygdbhhnbaeoq.png)
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
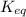
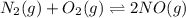
An equilibrium-constant expression for the equation is given by:
![K_(eq)=([NO]^2)/([N_2][O_2])](https://img.qammunity.org/2019/formulas/chemistry/college/ipsl1v22rc3t975zd5uni7ygdbhhnbaeoq.png)