Answer : There may be a mixture of products formed if the temperature is kept too warm, it will produce ortho, meta and para substituted nitro acetanilide.
Explanation : When acetanilide is treated with the nitrating mixture which the combination of
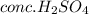 and
and
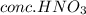 it undergoes nitration reaction. The temperature for this reaction must be maintained cold. The nitrating mixture is slowly added to the reaction mixture so that the nitronium ion concentration is low and also avoid polynitration of the ring. The cold temperatures is maintained to slow down the reaction rate which also help avoid over-nitration. If it gets too warm then there are many products formed along with the major para substituted nitro-acetanilide product. Substituted ortho and meta products contaminates the product. To avoid this cold temperature is required.
it undergoes nitration reaction. The temperature for this reaction must be maintained cold. The nitrating mixture is slowly added to the reaction mixture so that the nitronium ion concentration is low and also avoid polynitration of the ring. The cold temperatures is maintained to slow down the reaction rate which also help avoid over-nitration. If it gets too warm then there are many products formed along with the major para substituted nitro-acetanilide product. Substituted ortho and meta products contaminates the product. To avoid this cold temperature is required.