Hello!
To determine [H₃O⁺], we need to apply the Henderson-Hasselback equation, since this is a case of an acid and its conjugate base:
![pH=pKa+log( ([A^(-)] )/([HA]) )](https://img.qammunity.org/2019/formulas/chemistry/college/uvqofvmqo6bu9yhmtl8thntkpgwd6i9b57.png)
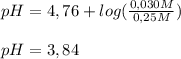
Now, we use the definition of pH and clear [H₃O⁺] from there:
![pH=-log[H_3O^(+)]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/pkepdmd3atxn89jucfz0hiy15yu35pw3gr.png)
![[H_3O^(+)] = 10^(-pH) =10^(-3,84)=0,00014 M](https://img.qammunity.org/2019/formulas/chemistry/college/kju54cgkkfwj2xs1576qvyzekupmkoxoml.png)
So, the [H₃O⁺] concentration is
0,00014 M
Have a nice day!