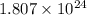Answer: The number of atoms in given amount of fluorine gas are
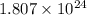
Step-by-step explanation:
We are given:
Moles of fluorine gas
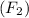 = 1.50 moles
= 1.50 moles
1 mole of fluorine gas contains 2 moles of fluorine atoms
According to mole concept:
1 mole of a substance contains
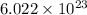 number of particles
number of particles
So, 1.50 moles of fluorine gas will contain
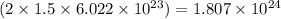 number of atoms.
number of atoms.
Hence, the number of atoms in given amount of fluorine gas are