Answer:
V = 93.0 L
Step-by-step explanation:
Hello!
In this case, when computing the PVT properties for an ideal gas, we need to use the following equation:

In such a way, for 2.5 x 10²⁴ molecules of chlorine at STP (273.15 K and 1.00 atm) we first need the moles based on the Avogadro's number:
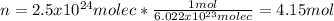
Now, we solve for volume on the ideal gas equation to obtain:
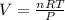
So we plug in and get:
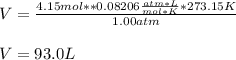
Best regards!