Answer:
B. -5.58°C.
Step-by-step explanation:
Hello!
In this case, since the freezing point depression is computed as follows:
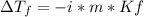
Whereas i=1 as the van't Hoff's factor of sugar (nonionizing solute), m=3mol/1kg=3mol/kg as the molality and Kf=1.86 °C/(mol/kg) as the freezing point depression constant for water. In such a way, we plug in to obtain:
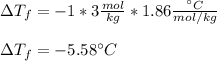
Now, since the freezing point of pure water is 0°C, we infer that freezing point of such solution is:
B. -5.58°C.
Best regards!