Answer 1 : The limiting reactant is, hydrogen gas,

Solution :
First we have to calculate the moles of hydrogen gas.

Now we have to calculate the volume of hydrogen gas.
As, 1 mole of gas contains 22.4 L volume of gas
So, 1.25 mole of hydrogen gas contains
 volume of hydrogen gas
volume of hydrogen gas
Now we have to calculate the limiting and excess reactant.
The given balanced reaction is,

From the balanced reaction, we conclude that
As, 44.8 L of hydrogen gas react with 22.4 L of carbon monoxide gas
So, 28 L of hydrogen gas react with
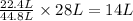 of carbon monoxide gas
of carbon monoxide gas
The excess carbon monoxide = 30 L - 14 L = 16 L
Thus, the carbon monoxide is an excess reactant because it is present in excess amount and hydrogen gas is a limiting reactant because it is present in limited amount.
Answer 2 : The mass of
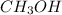 is, 20 grams
is, 20 grams
Solution :
From the balance reaction, we conclude that
2 moles of hydrogen gas react to give 1 mole of
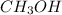
1.25 moles of hydrogen gas react to give
 mole of
mole of
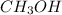
Now we have to calculate the mass of
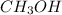


The mass of
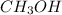 is, 20 grams
is, 20 grams
Answer 3 : The amount of excess reactant is, 20 grams
The excess reactant is carbon monoxide.
As, 22.4 L volume of carbon monoxide gas has 28 gram of carbon monoxide gas
So, 16 L volume of carbon monoxide gas has
 gram of carbon monoxide gas
gram of carbon monoxide gas
Thus, the The amount of excess reactant is, 20 grams