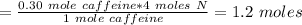Answer:
Moles of Nitrogen in 0.30 moles of caffeine is 1.2
Step-by-step explanation:
The molecular formula of caffeine is C8N4O2H10.
Based on the formula stoichiometry:
1 mole of caffeine contains-
8 moles of Carbon (C)
4 moles of nitrogen (N)
2 moles of oxygen (O)
10 moles of hydrogen (H)
Therefore, # moles of Nitrogen (N) in 0.30 moles of caffeine would be: