The radius of a nucleus of hydrogen is approximately
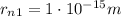
, while we can use the Borh radius as the distance of an electron from the nucleus in a hydrogen atom:
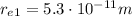
The radius of a dime is approximately
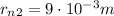
: if we assume that the radius of the nucleus is exactly this value, then we can find how far is the electron by using the proportion
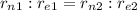
from which we find
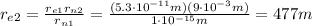
So, if the nucleus had the size of a dime, we would find the electron approximately 500 meters away.