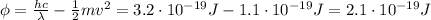In the photoelectric effect, part of the energy of the photon (E=hf) of the incoming light is used to extract the photoelectron from the metal (work function:
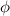
) and part is given to the electron as kinetic energy:
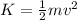
:
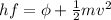
The frequency of the photon is related to the wavelenght:
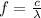
, and so we can re-write the formula as
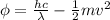
where:
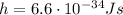
is the Planck constant
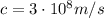
is the speed of light

is the wavelength of the light

is the electron mass
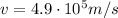
is the electron speed.
Substituting these numbers into the equation, we can find the work function of the surface: