Correct AnswerFourth Option, 2518 g
Solution:The formula of number of moles (n) in terms of mass of the compound(m) and molar mass of the compound (M) can be stated as:
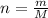
We have the number of moles of Octane.
So,
n = 22.05 moles
We have the molar mass of octane.
So,
M=114.22 g/mol
Using these values in the above formula we can calculate mass of Octane (m).
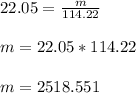
Therefore, the mass of 22.05 moles of Octane is 2518 g.