For Any gas:
P V = n R T
where:
P : pressure
V : volume
n : number of moles
R : gas constant
T : absolute temperature in kelvin
provided that:
V = 53 LS.T.P means standard temperature and pressure at which pressure is
1 atm and temperature is
273 K
R = 0.08205 atm.L /mol.K
So:
n =
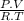
=
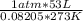
= 2.4 moles
The correct answer is A) 2.4