Answer:

Step-by-step explanation:
The reaction is a double decomposition, displacement or salt metathesis reaction. It is a chemical reaction between two compounds resulting in the interchange of one part of each to form two different compounds.
Hence both
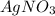 and NaCl exchange parts to form new products as follows:
and NaCl exchange parts to form new products as follows:

The correct equation showing all the phases of the reactants/products involved is
