Answer : 'Mg' being oxidized and reducing agent. 'Si' being reduced and oxidizing agent.
Step-by-step explanation:
Oxidation reaction : It is defined as the reaction in which a substance looses its electrons. In this, oxidation state of an element increases. Or we can say that in oxidation, the loss of electrons takes place.
Reduction reaction : It is defined as the reaction in which a substance gains electrons. In this, oxidation state of an element decreases. Or we can say that in reduction, the gain of electrons takes place.
The balanced chemical reaction is :
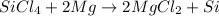
The half oxidation-reduction reactions are:
Oxidation reaction :
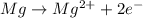
Reduction reaction :
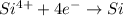
From this we conclude that the 'Mg' is the reducing agent that loses electron to another chemical species in a redox chemical reaction and itself get oxidized due to increase in the oxidation number from (0) to (+2).
'Si' is the oxidizing agent that gains electron from another chemical species in a redox chemical reaction and itself get reduced due to decrease in the oxidation number from (+4) to (0).
Hence, 'Mg' being oxidized and reducing agent. 'Si' being reduced and oxidizing agent.