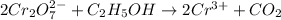Step-by-step explanation:
A redox equation is defined as an equation that shows reduction and oxidation are taking place simultaneously in a chemical reaction.
When electrons are lost in a chemical reaction then it is known as oxidation reaction. Whereas when electrons are gained in a chemical reaction then it is known as a redox reaction.
For example,
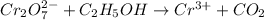
Reduction-half reaction:
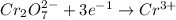
Oxidation state of Cr in
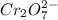 is +6.
is +6.
Oxidation-half reaction:
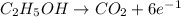
Oxidation state of C in
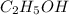 is -2 and in
is -2 and in
 oxidation state of C is +4.
oxidation state of C is +4.
Now, to balance the equation multiply reduction-half reaction by 2.
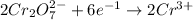
Thus, the combined balanced chemical reaction equation will be as follows.