Hello!
The net chemical equation between Sodium Bicarbonate and H₂SO₄ is the following one:
2NaHCO₃ + H₂SO₄ → Na₂SO₄ + 2H₂O + 2CO₂
To calculate the amount of Sodium Bicarbonate needed to neutralize 1000 L of 0,350 M H₂SO₄ we'll need to use the following conversion factor, to go from the volume of H₂SO₄ to grams of Sodium Bicarbonate:
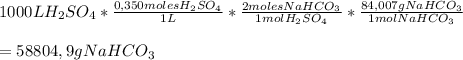
So, to neutralize 1000 L of 0,350 moles of H₂SO₄ you'll need
58804,9 grams of NaHCO₃