Answer: 2 electrons.Step-by-step explanation:The quantum numbers given are:
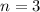
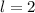
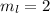
Paulis' exclusion principle states that only two electrons may occupy the same orbital, and that is only if each elecron has opposite spins.
The three first quantum numbers, as those given, determine an orbital. So, as Pauli's exclusion principle only two electrons in one atom can have that same set of quantum numbers.
One of the electrons will have (3, 2, 2, +1/2) and the other (3,2,2,-1/2). The four quantum numbers cannot be the same.