The correct answer is D. The law of conservation of mass states that in a system matter can neither be created or destroyed, hence atoms of each element on reactant side should equal atoms of each element on product side. In the unbalanced equation:
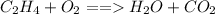 , the are 2 carbon atoms on reactant side as opposed to 1 carbon atom on product side, there are 4 hydrogen atoms on reactant side as opposed to 2 hydrogen atoms on product side, and there are 2 oxygen atoms on reactant side as opposed to 3 atoms of oxygen on product side. To balance the equation we add a coefficient of 3 on O_2 and on the product side we put a coefficient of 2 on both water
, the are 2 carbon atoms on reactant side as opposed to 1 carbon atom on product side, there are 4 hydrogen atoms on reactant side as opposed to 2 hydrogen atoms on product side, and there are 2 oxygen atoms on reactant side as opposed to 3 atoms of oxygen on product side. To balance the equation we add a coefficient of 3 on O_2 and on the product side we put a coefficient of 2 on both water
 and carbon dioxide
and carbon dioxide
 .
.
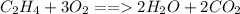 .
.