Hi!
The Dalton's Law of partial pressure states that T
he total pressure of a gas mixture is the sum of the individual pressures. This can be written in the form of an equation in the following way:
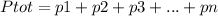
This law was first discovered by the English Chemist
John Dalton in 1802.
This law is derived from the observation that two gases in a mixture behave independently and occupy the same volume in a closed compartment, so the contribution to pressure will depend on the sum of the individual pressures of each gas.