Answer:
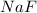
Step-by-step explanation:
When a weak acid and strong base reacts, it leads to the formation of a basic solution.
When a strong acid and weak base reacts, it leads to the formation of acidic solution.
When a strong acid and strong base or weak acid and weak base reacts, it leads to the formation of a neutral solution.
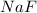 is made up of strong base
is made up of strong base
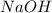 and a weak acid [tex}HF[/tex] , thus leading to formation of basic solution.
and a weak acid [tex}HF[/tex] , thus leading to formation of basic solution.