Answer:
![[C_6H_5COOH]_(eq)=0.06104M](https://img.qammunity.org/2019/formulas/chemistry/college/4tqp8fhuv15wy6j575zon0xdarracccxt5.png)
Step-by-step explanation:
Hello,
In this case, we properly write the undergoing dissociation reaction as follows:
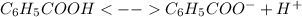
Know, by applying the law of mass action over this dissociation reaction, one obtains:
![Ka=([C_6H_5COO^-]_(eq)[H^+]_(eq))/([C_6H_5COOH]_(eq))](https://img.qammunity.org/2019/formulas/chemistry/college/hmobse0ubi34v7e7pewx1avd1il0x4kits.png)
Now, we have to notice that the equilibrium concentrations are given by the change
 , which alters the aforesaid equation in the following way, based on the ICE method:
, which alters the aforesaid equation in the following way, based on the ICE method:
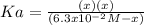
Now, solving for
 , we've got:
, we've got:
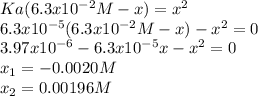
Finally, one computes the concentration of benzoic acid as;
![[C_6H_5COOH]_(eq)=0.0063M-0.00196M=0.06104M](https://img.qammunity.org/2019/formulas/chemistry/college/3p6d5wznt3d3hclpamcazebv639b93qb2y.png)
Best regards.