Let's convert the wavelength into meters first:
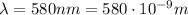
Then we know that the frequency f is related to the wavelength by the following relationship:
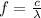
where
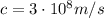
is the speed of light. Substituting the numbers, we get the frequency:
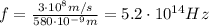
So, this is approximately the frequency of yellow light.